| 1.Inquiries will be replied within 24 hours |
| 2.We could supply various packages as you required |
| 3.To protect the profit of our agents, price will not show on website, please send inquiries to get the price. |
| 4.Fast delivery, goods arrive your office within 3 to 5 days |
| 5.Please click "Inquiry" or "Email" below to get the price |
|
|
|
|
|
|
|
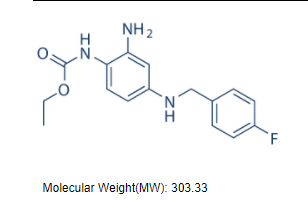
C16H18FN3O2 |
|
|
|
|
303.33 |
|
in stock |
|
|
150812-12-7 |
|
98%+ |
Introduction
Retigabine is a novel antiepileptic drug whose mechanism of action involves potassium channel opening activity in neuronal cells. Retigabine can markedly enhance KCNQ2/Q3 currents. In addition, retigabine also enhances slow channel deactivation. Retigabine has been shown to increase the synthesis of GABA in rat hippocampal slices and to enhance GABA-induced chloride currents in cultured rat cortical neurons. Retigabine has been shown to induce membrane hyperpolarization in neurones in rat hippocampal-entorhinal cortex slices and to exert potassium channel opening activity in neuronal cells. Retigabine enhances a linopirdine-sensitive current in differentiated PC12 cells. Concentrations of RTG/EZG ≥10 μM are required to cause significant augmentation of the GABAA receptor response. The potency of RTG/EZG differed somewhat depending on the GABAA receptor subunit combination, with the following rank order: α1β3γ2=α1β2γ2 >α3β2γ2=α2β2γ2>α5β2γ2=α1β2(N265S)γ2=α1β1γ2. RTG/EZG exhibits only weak inhibitory effects at voltage-gated Nav and Cav channel currents at predominantly supratherapeutic concentrations. RTG/EZG does not interact significantly with glutamate receptors
Retigabine enhances γ-aminobutyric acid (GABA)-ergic transmission in the central nervous system. Retigabine is rapidly absorbed and distributed with an oral bioavailability of 60% and a high volume of distribution of approximately 6.2 L/kg. Tolerability is good in humans when titrated up to its therapeutic dose range (600-1200 mg/day). Plasma protein binding of the drug is approximately 80%. The relatively high systemic bioavailability after oral administration suggests that retigabine is resistant to first-pass metabolism, a finding confirmed in multiple species. Retigabine is metabolized exclusively via phase II hepatic glucuronidation and acetylation. Gender differences in exposure have been noted, with female subjects exhibiting higher plasma concentrations of the drug after oral administration than male subjects. Excretion of retigabine appears to be predominantly renal. Depending on the behavioral endpoints analyzed, it appears that retigabine has a relatively poor therapeutic index (ratio between TD50 obtained in rotarod and ED50 obtained in maximal electroshock where maximal tonic extension of the hindlimbs was used as endpoint) in both mice (TD50/ED50 = 2.2) and rats (TD50/ED50 = 1.9) after i.p. administration. However, after p.o. administration in rats retigabine shows a therapeutic index of 28.8, which compares favorably with that reported for other antiepileptics, such as carbamazepine
Products for scientific research use only