Products
Milciclib 802539-81-7
| 1.Inquiries will be replied within 24 hours |
| 2.We could supply various packages as you required |
| 3.To protect the profit of our agents, price will not show on website, please send inquiries to get the price. |
| 4.Fast delivery, goods arrive your office within 3 to 5 days |
| 5.Please click "Inquiry" or "Email" below to get the price |
 |
|
|
||||||
|
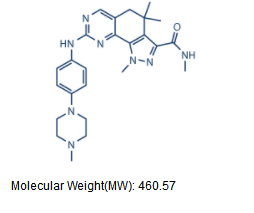
C25H32N8O |
|
|||||
|
460.57 |
|
in stock | ||||
|
802539-81-7 |
|
98%+ |
Introduction
Milciclib (PHA-848125) is a potent, ATP-competitive CDK inhibitor for CDK2 with IC50 of 45 nM. It is >3-fold more selective for CDK2 than CDK1, 2, 4, 5, and 7
PHA-848125 inhibits, although with lower potency, the activities of cyclin H/CDK7, cyclin D1/CDK4, p35/CDK5, as well as cyclin E/CDK2 and cyclin B/CDK1 with IC50 values of 0.15, 0.16, 0.265, 0.363, 0.398 μM, respectively. Thropomyosin receptor kinase A is also inhibited by PHA-848125 in the same nanomolar range as CDKs. In the most PHA-848125-sensitive cell line, PHA-848125 induces a concentration-dependent G(1) arrest. PHA-848125 also impairs phosphorylation of the retinoblastoma protein at CDK2 and CDK4 specific sites, reduces retinoblastoma protein and cyclin A levels, and increases p21(Cip1), p27(Kip1) and p53 expression. PHA-848125 is added to the cells 48 h after TMZ and cell growth is evaluated after 3 additional days of culture. A drug combination of TMZ, BG and PHA-848125 induces an additive or synergistic effect on cell growth, depending on the cell line. In the absence of BG, the combination is still more active than the single agents in cell lines moderately sensitive to TMZ, but comparable to PHA-848125 alone in the two TMZ-resistant cell lines. When TMZ plus BG are used in combination with PHA-848125 against cultured normal melanocytes, neither synergistic nor additive antiproliferative effects are observed.
Products for scientific research use only
PHA-848125 inhibits, although with lower potency, the activities of cyclin H/CDK7, cyclin D1/CDK4, p35/CDK5, as well as cyclin E/CDK2 and cyclin B/CDK1 with IC50 values of 0.15, 0.16, 0.265, 0.363, 0.398 μM, respectively. Thropomyosin receptor kinase A is also inhibited by PHA-848125 in the same nanomolar range as CDKs. In the most PHA-848125-sensitive cell line, PHA-848125 induces a concentration-dependent G(1) arrest. PHA-848125 also impairs phosphorylation of the retinoblastoma protein at CDK2 and CDK4 specific sites, reduces retinoblastoma protein and cyclin A levels, and increases p21(Cip1), p27(Kip1) and p53 expression. PHA-848125 is added to the cells 48 h after TMZ and cell growth is evaluated after 3 additional days of culture. A drug combination of TMZ, BG and PHA-848125 induces an additive or synergistic effect on cell growth, depending on the cell line. In the absence of BG, the combination is still more active than the single agents in cell lines moderately sensitive to TMZ, but comparable to PHA-848125 alone in the two TMZ-resistant cell lines. When TMZ plus BG are used in combination with PHA-848125 against cultured normal melanocytes, neither synergistic nor additive antiproliferative effects are observed.
Products for scientific research use only